About us

KEURO Solutions
KEURO Solutions is an Asia-Pacific based contract manufacturer with experience and focus on interventional medical devices.
With an excellent team of R&D engineers, production workers and RA/QA professionals, we are here to help our clients in the Medtech industry from early-stage start-ups all the way through large volume manufacturing. We are committed to the highest quality standards, shortest lead-times, and flexibility to meet our customer’s needs.

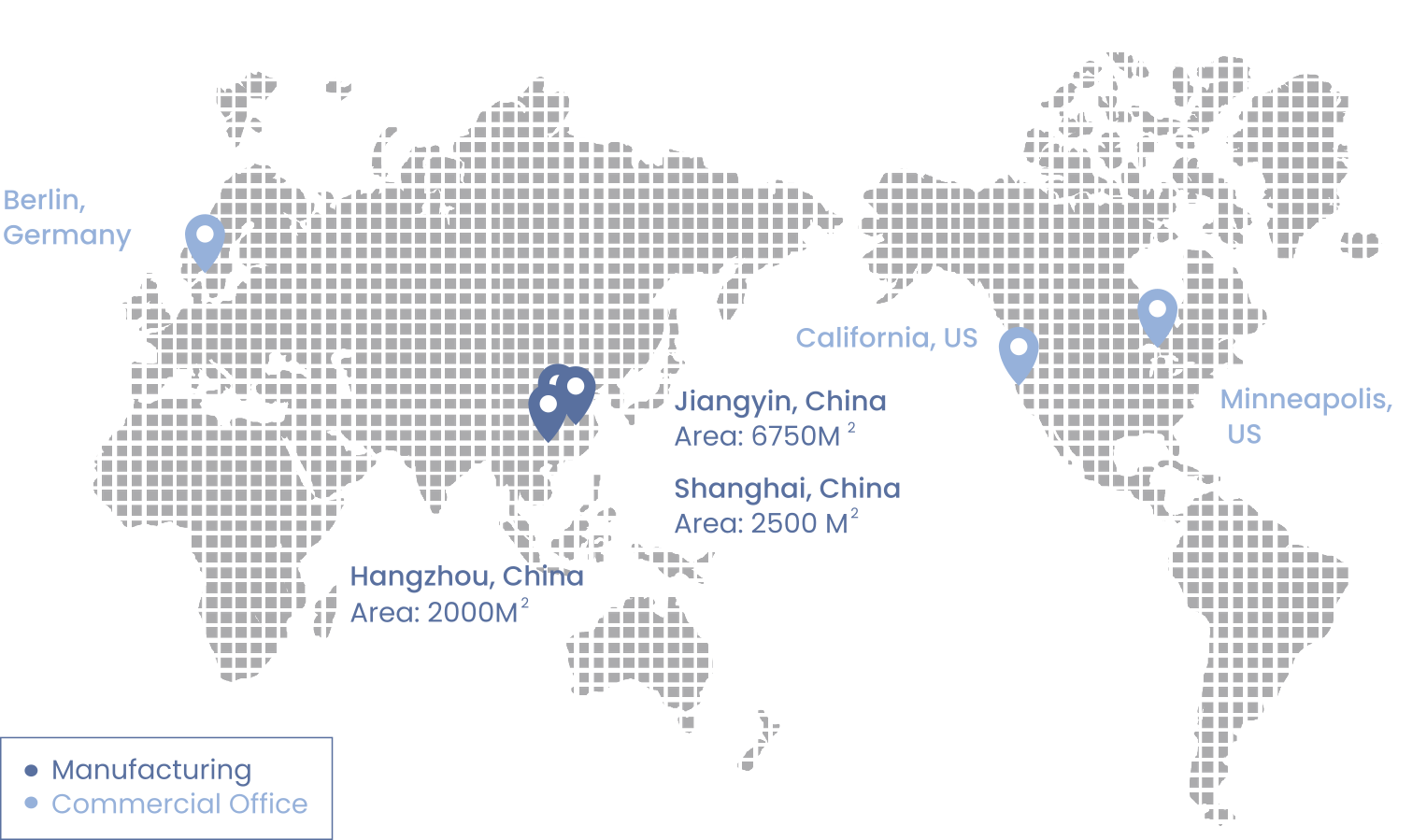
Our Facilities

Certified Company
Quality Control
Keuro Soultions has solid expertise in the medical device complex regulatory environment. We design and manufacture medical devices in full compliance with US FDA, European MDR and China NMPA guidelines. Our manufacturing facilities are ISO 13485 certified.
Service Commitment

Top Quality Control
ISO 13485 certified facilities

Flexible to Customers' Needs
We tailor our processes and resources to meet your specific requirements at every stage.

Short Lead Time
Streamlined processes and in-house capabilities enable faster, reliable delivery